Proxima Clinical Research Client, Airway Shield™, Receives STeP Designation for Endotracheal Intubation Device that Protects Patients and Clinicians
ACCESS Newswire
18 May 2022, 21:19 GMT+10
Device reduces the spread of infection from patients to clinicians during aerosol generating procedures like intubation of COVID-19 patients, among others
HOUSTON, TX / ACCESSWIRE / May 18, 2022 / Proxima Clinical Research ('Proxima CRO'), a company guiding emerging medical device and pharmaceutical companies from the earliest stages of product development through commercialization, announced today it has successfully received Safer Technologies Program ('STeP') Medical Device Designation for its client, Airway Shield™. Airway Shield is a mouthpiece used during endotracheal intubation ('ET intubation') that facilitates the insertion of an ET tube and serves as a barrier during aerosol generating procedures to eliminate the spread of aerosols and droplets emitted by the patient to medical staff, thereby reducing the risk of infections and spread of viruses such as COVID-19.

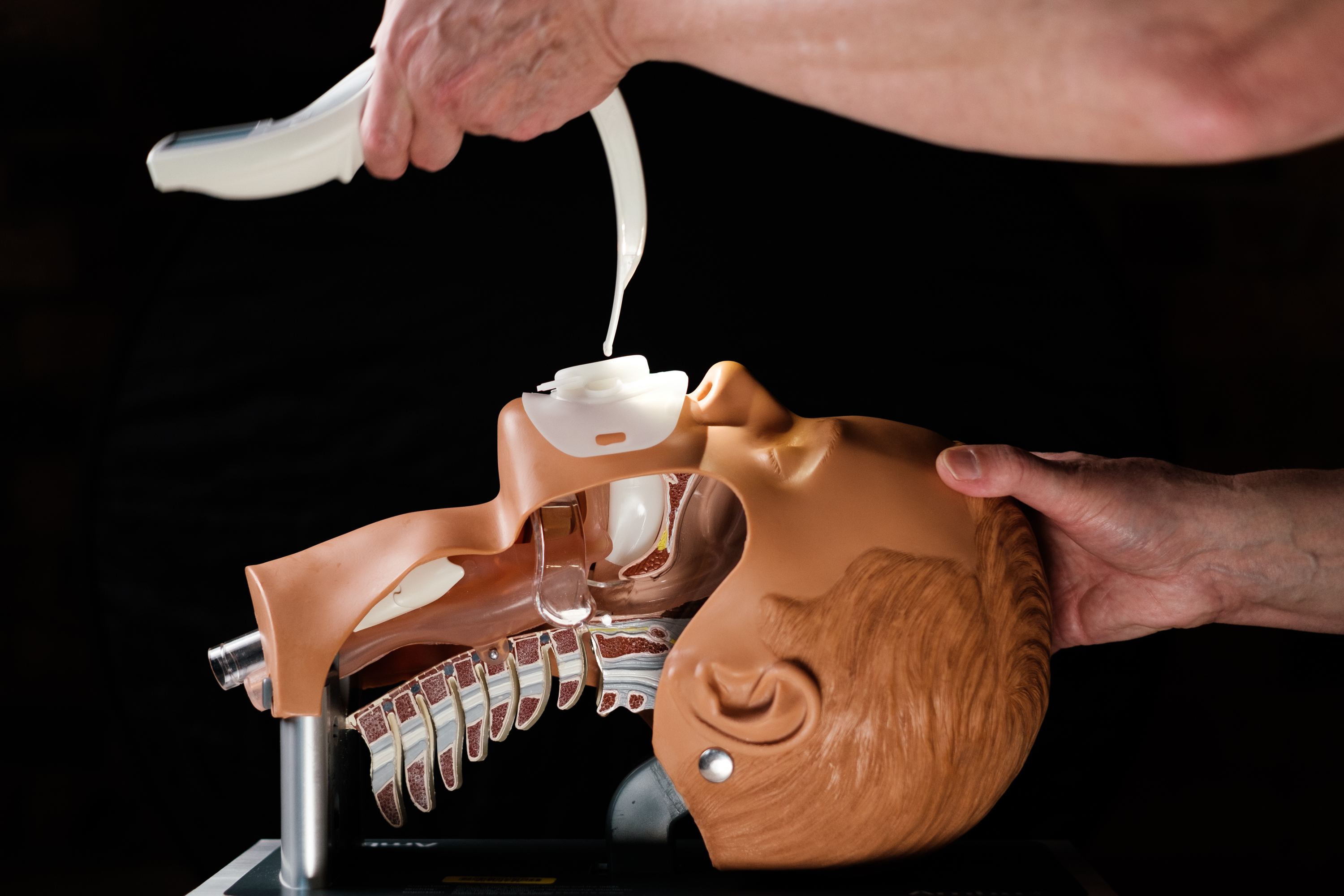
ET intubation is an aerosol generating procedure with a high risk of infection for operators. The procedure permits mechanical ventilation in patients with severe respiratory failure, such as many patients hospitalized with COVID-19, among other conditions, and is required in many surgeries. It is the third most common procedure in U.S. hospitals with more than 50 million intubations performed worldwide.
The Safer Technologies Program ('STeP') is a voluntary program for certain medical devices and device-led combination products that are reasonably expected to improve the safety of current treatments or diagnostics associated with less serious morbidities and mortalities than those eligible for the Breakthrough Device Designation ('BDD') program. Unlike BDD, STeP eligible devices may include those intended to treat or diagnose non-life-threatening or reasonably reversible diseases or conditions.
'The benefits of STeP are significant because the program expedites the time it takes to develop and obtain marketing authorization for devices,' said Isabella Schmitt, RAC, Director of Regulatory Affairs at Proxima CRO. 'Airway Shield receives this designation as the world still struggles with COVID-19 infections. WHO estimates that between 80,000 and 180,000 health and care workers may have died from COVID-19 between January 2020 to May 2021. The Airway Shield disposable device makes intubation safer for both the patient and clinical staff. It is easy to operate with minimal training and is highly cost-effective. We are pleased to have received this important designation for Airway Shield.'

Airway Shield™ is the world's first device for endotracheal intubation that protects clinicians while facilitating ET intubation procedures. Its patented technology incorporates a barrier mouthpiece that protects clinicians from aerosols and droplets that could spread to the operator and around the room while facilitating the procedure. The device acts as a guide, enabling faster, more accurate positioning of the endotracheal tube. It also features two extra channels which permit oropharyngeal aspiration during the procedure. The device is fully disposable and quick and easy to remove, which reduces the risk of transmission during removal.
'This is a groundbreaking accomplishment for our team,' says Julio Alonso, MD, founder and CEO, Airway Shield. 'Our collaboration with Proxima CRO and our partners, trucorp and CSDS, confirms our commitment to protect the lives of patients and clinicians throughout the world. Our team is looking forward to working with the FDA and Proxima CRO through the interactions available with the STeP designation to quickly bring this product to market.'
About Airway ShieldTM
At Airway Shield, we strive to provide a solution that minimizes the risk of transmission of aerosol-borne pathogens during endotracheal intubation. Our aim is to fill the failure gap of current personal protective equipment while enabling clinicians to secure the airway with first pass success. Although Airway Shield was initially conceived as a response to COVID-19, increased scientific awareness about the risks of aerosol transmission points to an important role for this technology in critical patients with other common, potentially life-threatening respiratory infections. Airway Shield is the world's first device for endotracheal intubation that protects clinicians while facilitating the procedure. The device has not yet been reviewed by FDA and is not available for sale in the US. For more information, visit airwayshield.com.
About Proxima Clinical Research
Proxima CRO provides regulatory and clinical research expertise to life sciences companies of all sizes and stages, including emerging companies, inventors, and Fortune 500. With headquarters in the Texas Medical Center ('TMC'), the largest medical center in the world, Proxima CRO brings its expertise to hundreds of medical device, pharmaceutical, biotechnology, and diagnostic companies in 17 countries across five continents to further advance the $130 billion industry. Launched in November 2017, Proxima CRO is a registered Delaware C Corporation. For more on Proxima CRO, visit ProximaCRO.com.
Media Contact:
Jennifer Horspool
(949)-933-4300
[email protected]
SOURCE: ProximaCRO
View source version on accesswire.com:
https://www.accesswire.com/701885/Proxima-Clinical-Research-Client-Airway-Shield-Receives-STeP-Designation-for-Endotracheal-Intubation-Device-that-Protects-Patients-and-Clinicians
 Share
Share
 Tweet
Tweet
 Share
Share
 Flip
Flip
 Email
Email
Watch latest videos
Subscribe and Follow
Get a daily dose of Dallas Sun news through our daily email, its complimentary and keeps you fully up to date with world and business news as well.
News RELEASES
Publish news of your business, community or sports group, personnel appointments, major event and more by submitting a news release to Dallas Sun.
More InformationInternational
SectionWhite House meeting between Trump, Netanyahu on July 7
WASHINGTON, D.C.: President Donald Trump will meet Israeli Prime Minister Benjamin Netanyahu at the White House on Monday. President...
Over 60 companies named in UN report on Israel-Gaza conflict
GENEVA, Switzerland: A new United Nations report alleges that dozens of global corporations are profiting from and helping sustain...
UK lawmakers desigate protest group as terrorist organization
LONDON, UK - Lawmakers in the United Kingdom have voted overwhelmingly to proscribe the direct-action group Palestine Action as a terrorist...
Dalai Lama to address Buddhist conference, reveal succession plan
DHARAMSHALA, India: The Dalai Lama is set to address a significant three-day conference of Buddhist leaders this week, coinciding with...
US Supreme Court backs Texas efforts to shield minors online
WASHINGTON, D.C.: In a significant ruling last week, the U.S. Supreme Court upheld a Texas law requiring age verification for users...
Turkey, France battle wildfires amid early Europe heatwave
ISTANBUL/PARIS/BRUSSELS: As searing temperatures blanket much of Europe, wildfires are erupting and evacuation orders are being issued...
Business
SectionGrammarly acquires Superhuman to boost AI workplace tools
SAN FRANCISCO, California: Grammarly is doubling down on AI-powered productivity tools with the acquisition of Superhuman, a sleek...
Standard and Poor's 500 and and Nasdaq Composite close at record highs
NEW YORK, New York -U.S. stock markets closed with broad gains on Thursday, led by strong performances in U.S. tech stocks, while European...
Persson family steps up H&M share purchases, sparks buyout talk
LONDON/STOCKHOLM: The Persson family is ramping up its investment in the H&M fashion empire, fueling renewed speculation about a potential...
L'Oreal to buy Color Wow, boosts premium haircare portfolio
PARIS, France: L'Oréal is making a fresh play in the booming premium haircare segment with a new acquisition. The French beauty conglomerate...
Robinhood launches stock tokens for EU investors, adds OpenAI
MENLO PARK, California: Robinhood is giving European investors a new way to tap into America's most prominent tech names — without...
Wall Street diverges, but techs advance Wednesday
NEW YORK, New York - U.S. stocks diverged on Wednesday for the second day in a row. The Standard and Poor's 500 hit a new all-time...